Medical Devices
Medical Devices
PolyAnalytik’s breakthrough validated methods have led to successful molecular degradation studies and complete molecular analyses, saving sponsors millions of dollars and time in their approval process in the US-FDA and around the world, as well as adding competitive advantage on the performance and safety of their products. Since the first development of synthetic poly(glycolic acid) based suture system in the 1960s, synthetic biodegradable polymers, with uniform molecular structure and controllable properties have become the major biomaterials used in medical devices such as scaffolds, implants, drug delivery vectors, and sutures (Figure 1.1., Table 1.1).
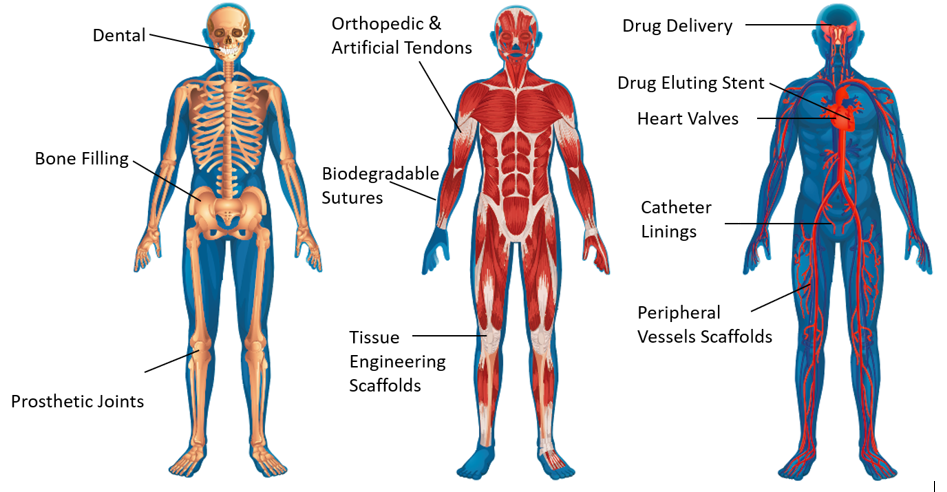
Figure 1.1. Medical device therapies.
Table 1.1. Medical device therapies.
-
- Skeletal
- Musculoskeletal
- Cardiovascular
-
- Dental
- Orthopedic & artificial tendons
- Drug delivery
-
- Bone filling
- Biodegradable sutures
- Drug eluting stent
-
- Prosthetic joints
- Tissue engineering scaffolds
- Heart valves
-
- --
- --
- Catheter linings
-
- --
- --
- Peripheral vessels scaffolds
In 2005, approximately 445,687 patients died from coronary artery disease (CAD) in the United States accounting for 20% of all deaths that year. Over the past two decades, percutaneous transluminal coronary angioplasty (PTCA) with bare-metal stents (BMS) has been used as a treatment for obstructive CAD (Figure 1.2). However, a high percentage of patients experience arterial narrowing or restenosis resulting in the need for reintervention.
Recently, DES have been developed to address the drawbacks associated with using BMS and to promote healing of the vessel after opening the blockage. DES consists of a BMS platform coated with an antiproliferative drug-polymer film. The first generation of DES were found to be associated with an increased risk of sub-acute and delayed stent thrombosis (ST). Although the etiology of stent thrombosis has yet to be fully elucidated, studies have indicated that durable polymers used as part of the drug elution matrix can play a role by triggering inflammation and hypersensitivity reactions predisposing a patient towards ST.
Consequently, to avoid this complication, the next generation DES utilizes biodegradable polymers either as part of the drug-matrix applied to the stent and/or as a replacement for the metal scaffold. Given the association between polymer presence and ST, next generation DES have shifted to use of faster resorbing polymers to reduce the risk of ST.
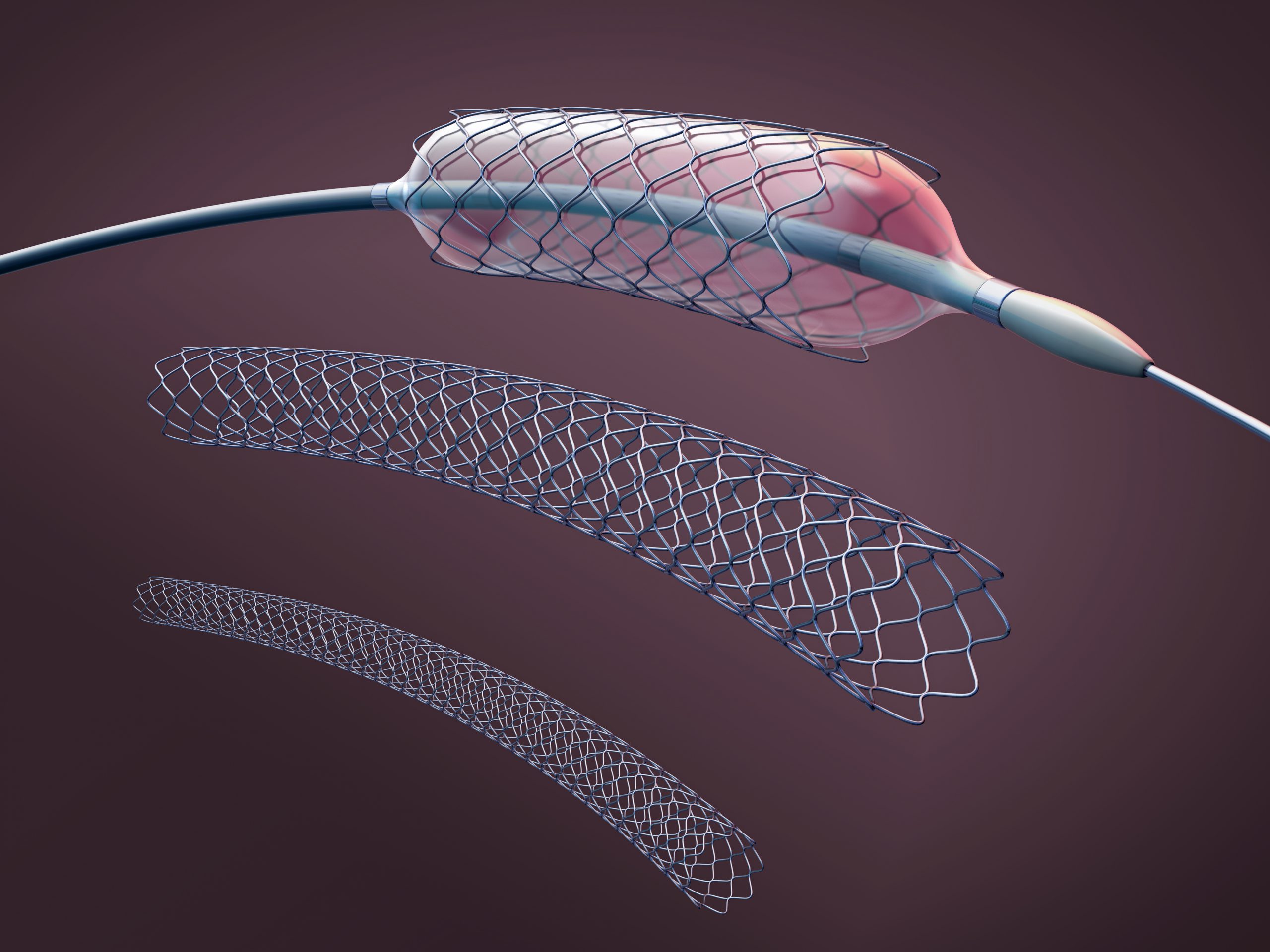
Figure 1.2. Bare metal stent (BMS), often used to treat coronary artery disease (CAD).
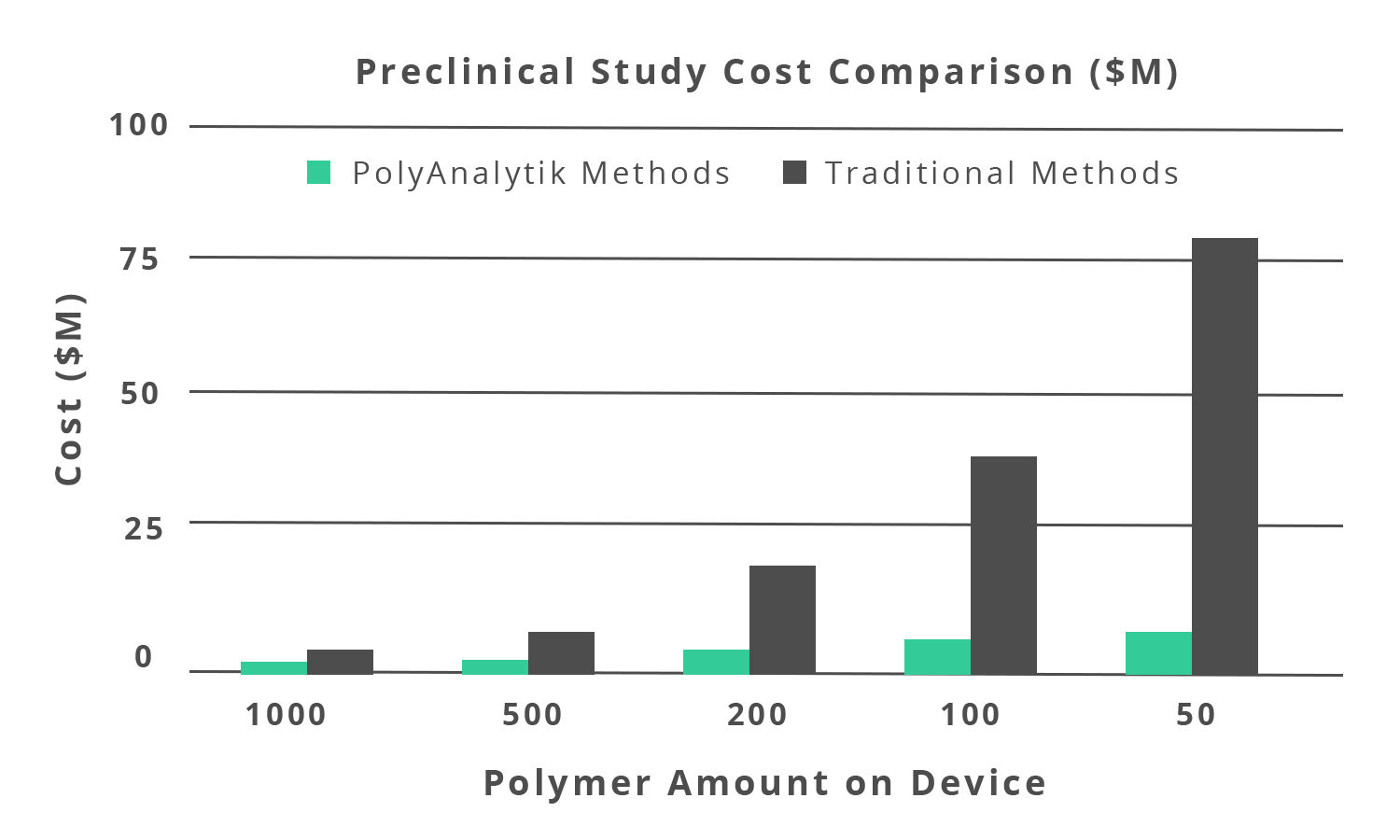
The potential clinical impact of polymer absorption rate underscores the need for a robust methodology to assess polymer degradation in vitro and, particularly, in vivo to ensure devices are designed with the appropriate profiles. It was found in a bioanalysis (in vivo) and a bench-top (in vitro) degradation assessment, that the polymer GPC elution profile decreased over time and how the drug eluted from the combined arterial tissue and stent. The correlation of the polymer drug coating performance was also established by incubating the devices in a buffer and measuring the degradation kinetics of the coating (i.e. in vitro). With this key characterization, the sponsor of this DES was able to obtain US-FDA clearance to begin clinical trials.Studies should not have to sacrifice innovation due to cost barriers. The in vivo and in vitro degradation assessment methods developed by PolyAnalytik save our clients millions of dollars in preclinical study costs by drastically reducing the number of devices and animals required for studies (Figure 1.3). With our methods, we aim to facilitate life saving medical innovations for people worldwide.